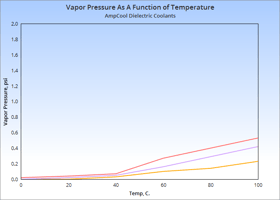
If you're designing a closed-loop cooling system, be sure to consider the Vapor Pressure of the...
Why SLIC Cools Better Than Air:
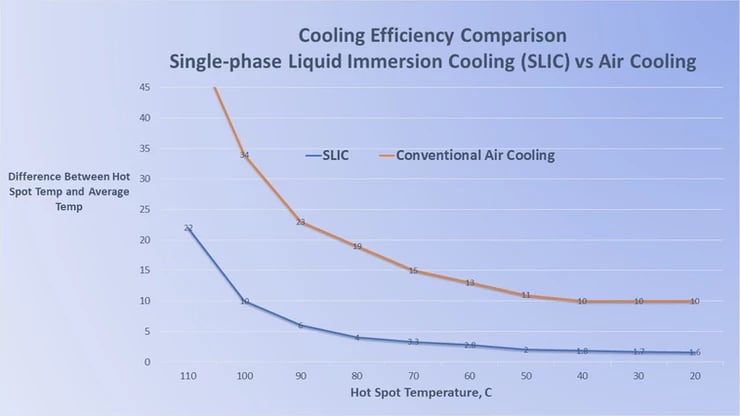
SLIC: 1400X More Efficient Than Air Cooling
So a customer asked me last week if electronics assemblies could “feel the difference” between cooling with air and with SLIC (Single-phase Liquid Immersion Cooling) Technology. I thought that it was an interesting question that would be helpful for everyone to understand.
The phenomenon of “feeling” different at the same temp is really the physical manifestation of a material characteristic called “heat capacity”. Think of it as “thermal mass” and the ability to “suck heat out of something”.
Technically, “heat capacity” is the amount of energy that you have to put into something in order to raise its temperature 1 degree. It’s different for different materials. A low heat capacity means that something gets hot with very little energy input – there’s little energy stored in that hot material. A material with high heat capacity gets hotter more slowly – it’s absorbing a lot of energy, and can hold it when the material is hot.
Some analogies are helpful: Let’s imagine standing next to a swimming pool and that both the air and the pool water are at 60 degrees F. As you stand in 60 degree air, it’s chilly, but since air doesn’t have much heat capacity, the cool air doesn’t “suck the heat out of you” very quickly, and your body can generate enough heat to keep you warm. If you jump into a 60 degree pool, though, the water has far higher heat capacity, and will cool you down to 60 much more quickly – quicker, for example, than your body can generate heat. Now, just because the heat capacity is high doesn’t mean that it can lower your body temp below 60 (you body’s never going to go to 50 in this scenario), but it’ll get to 60 very quickly. Dangerously quickly.
I often use the analogy of sticking your arm into a 400 degree oven. You can do that, it feels hot, but it doesn’t hurt your arm. But the minute you touch the baking rack, which is also at 400 degrees, then you’re burned. That’s because the steel rack has far higher heat capacity than the air, and “holds” more heat that then burns you when you touch it.
So, electronics assemblies don’t get any cooler while immersed in a liquid coolant than it does in the same temperature air, but the coolant’s high heat capacity allows it to “suck the heat out” much more quickly than air can. The rapidity at which the heat is removed from the electronics doesn’t have any bad effects, in fact it keeps the temperature very constant as the electronics heats up.